iPledge (Risk Evaluation and Mitigation Strategy): Certain drugs cause side effects when administered to patients. For instance, Isotretinoin is issued by wholesale dealers, and pharmacies provided the patient produces a prescription. The drug is used for mild and moderate acne to patients but when it is used on pregnant women it may cause a high risk to newly born infants. Likewise, one can find other drugs such as Claravis, Amnesteem, and Myorisan. These drugs enter the pharma markets as Absorica and Zenatane. These drugs can be even found in markets with a former brand name as Accutane.
Realizing the sensitivity of the drug, the United States Food and Drugs Administration (FDA) has introduced iPLEDGE (risk evaluation and mitigation strategy) to govern the possible risk of conceiving a baby with birth defects. In which, the United States Food & Drug Administration (FDA) has devised and developed an app and it is made compulsory to register the sold drug by wholesale dealers and pharmacies. The entire iPledge program app has been designed by the Isotretinoin Product Manufacturers Group, IPMG, as per the instructions of the FDA. In fact, the program app serves the purpose of the women who use isotretinoin prescriptions be it pregnant or unpregnant ones. The app keeps an updated record of the patient’s health conditions after administering isotretinoin products.
iPledge REMS Program Caution:
Isotretinoin products can create severe health conditions externally/ Internally to infants. To detail it, a baby can develop life-threatening internal problems and they can be brain damage, dysfunctioning of speech, walk, think or breathe. Further, it can lead to heart issues or severe intellectual disability. Some of the external problems a baby can confront are abnormally shaped skulls, disfigurement of face, eye abnormalities, cleft palate, and absence or small ear canals.
Read/Sign the Patient Information/Informed Consent:
- When a woman approaches a healthcare provider, she will be given a brief up about the usage of isotretinoin. Only after the consent of the woman, she will be asked to review and sign up the documentation about consent.
- The consent page contains full information to the patient in regard to isotretinoin. The information includes how it works, side effects, and the patient’s responsibility while getting the isotretinoin administered.
Patient Must Submit Regular Pregnancy Test:
1. The patient (woman) must pass two negative pregnancy tests and then enter to take isotretinoin for the first month.
2. The first month’s pregnancy test is conducted by the patient’s healthcare provider after being ascertained to get into the program.
3. Then, the Clinical Laboratory Improvement Amendments-certified Laboratory (CLIA) shall conduct the second test.
4. Each month, the patient must get a negative pregnancy result. The same should continue for the last pregnancy test that is conducted a month later after stopping the treatment.
5. You will also need a negative pregnancy test each month before getting your refill, and one last pregnancy test one month after stopping treatment.
6. The patient’s healthcare provider shall provide the patient with a list of the approved labs. The patient can opt to get the tests conducted from a CLIA-certified lab.
Two Forms of Contraception to be Used All Times:
1. The patient (woman) and the man must use two forms of contraception all time.
2. The two forms of contractive must be utilized a month before receiving the medication. Then, continue the same during the medical treatment, and for another month after stopping the treatment.
Caution: Before beginning with different forms of birth control, one must seek approval from the iPledge program.
Patients on Isotretinoin & Seek Healthcare Support, Every Month: The patient must approach the healthcare service provider and discuss the health issues and any side effects if one experiences them. One may be advised to take blood tests if needed.
Patient Must Collect Prescription Within 7-day Window:
- After the negative pregnancy test result, the patient must collect the prescription within seven days.
- In case one fails to obtain it, then the entire process to qualify for the pregnancy test should be done, and only then proceed to collect the prescription.
Caution:
- The patient who fails to collect the prescription within 7 days will have to wait for 19 days and then start the qualifying processes.
- During the 19-day haul, the healthcare provider and iPledge will lock the service for the patient.
- The user is advised to report if an adverse event occurs or in relation to pregnancy at 1-866-495-0654.
Patients Login at ipledgeprogram.com:
1st step: First, the Patients should Enter the web portal i.e. www.ipledgeprogram.com, and click the enroll/ login Option.

2nd Step: The Registered users need to fulfill the user name/ Email Address and hit the Login option. Further, the Users need to complete the login form by entering the Password and make sign-in it online.

3rd Step: The Web portal of ipledge shall lead to the patient’s logged-in web page.
New Users Enrollment process:
1. If the Users need to enroll on the Ipledge page should visit the link https://ipledgeprogram.com/#Main/Login and tap on the Prescriber or Pregnancy Options as shown below.
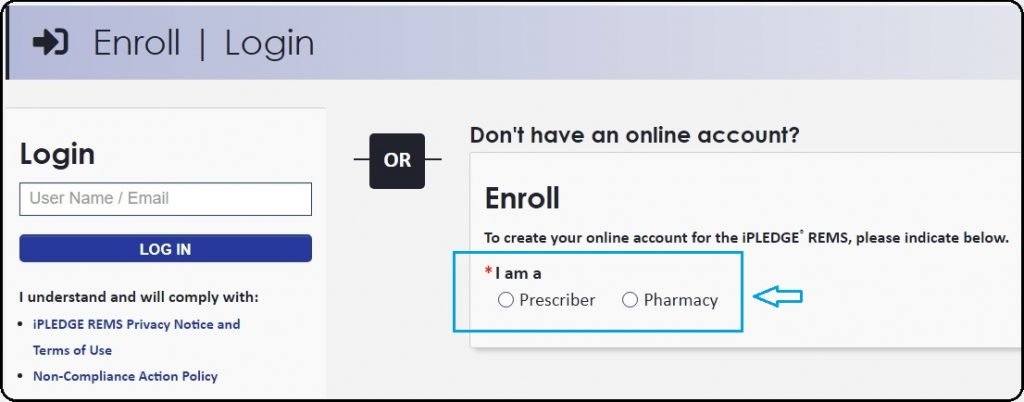
2. The Users can select wishing options and complete the enrollment.
Also Read: Myufhealth mychart Login
